Availability of take-home naloxone product approved by five European countries
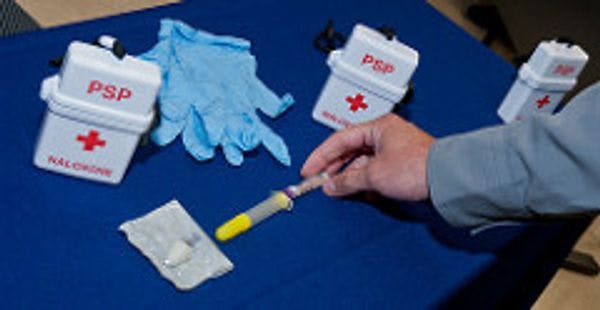
Prenoxad Injection, the product that is used as take-home naloxone, has achieved regulatory approval in Sweden, Denmark, Ireland, Finland and Estonia.
The medicine is designed for use by non-healthcare professionals to temporarily reverse the effects of opioid overdoses. The product is available in the UK and over 85,000 units or kits have been supplied to people who are at risk of opioid related overdose or to their nominated representative.
The Scottish National Naloxone Programme, an initiative that is led by SDF, has advocated the use of the medicine since the programme’s launch in 2010.
According to the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) approximately 1.3 million people in Europe suffer from opioid addiction. Evidence shows that accidental overdose is a leading cause of death in drug users, with around 6,500 men and women dying of an overdose each year across Europe.
Click here to read the full article.
Keep up-to-date with drug policy developments by subscribing to the IDPC Monthly Alert.
Thumbnail: Flickr CC Governor Tom Wolf